| Desc: |
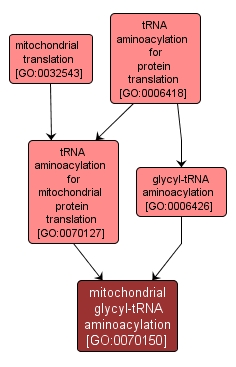
The process of coupling glycine to glycyl-tRNA in a mitochondrion, catalyzed by glycyl-tRNA synthetase. In tRNA aminoacylation, the amino acid is first activated by linkage to AMP and then transferred to either the 2'- or the 3'-hydroxyl group of the 3'-adenosine residue of the tRNA. |