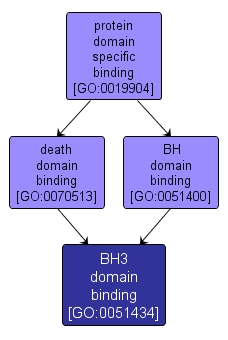
GO TERM SUMMARY
|
| Name: |
BH3 domain binding |
| Acc: |
GO:0051434 |
| Aspect: |
Molecular Function |
| Desc: |
Interacting selectively and non-covalently with the BH3 domain of a protein of the Bcl-2 family. The BH3 domain is a potent death domain and has an important role in protein-protein interactions and in cell death. |
|

|
INTERACTIVE GO GRAPH
|