GO TERM SUMMARY
|
| Name: |
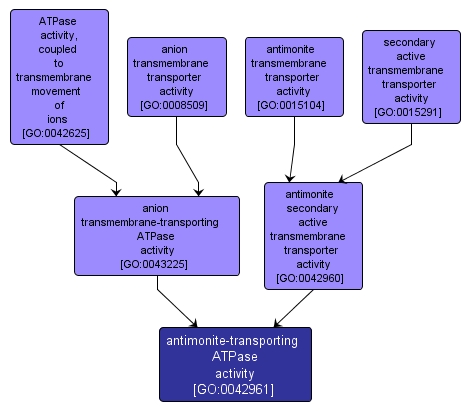
antimonite-transporting ATPase activity |
| Acc: |
GO:0042961 |
| Aspect: |
Molecular Function |
| Desc: |
Catalysis of the transfer of a solute or solutes from one side of a membrane to the other according to the reaction: ATP + H2O + antimonite(in) = ADP + phosphate + antimonite(out). |
Synonyms:
- antimonite ABC transporter
|
|

|
INTERACTIVE GO GRAPH
|