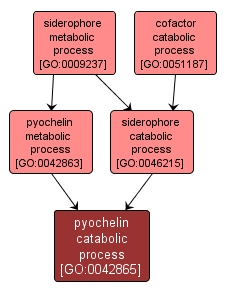
GO TERM SUMMARY
|
| Name: |
pyochelin catabolic process |
| Acc: |
GO:0042865 |
| Aspect: |
Biological Process |
| Desc: |
The chemical reactions and pathways resulting in the breakdown of the siderochrome pyochelin (2-(2-o-hydroxyphenyl-2-thiazolin-4-yl)-3-methylthiazolidine-4-carboxylic acid). |
Synonyms:
- pyochelin catabolism
- pyochelin degradation
- pyochelin breakdown
|
|

|
INTERACTIVE GO GRAPH
|