| Desc: |
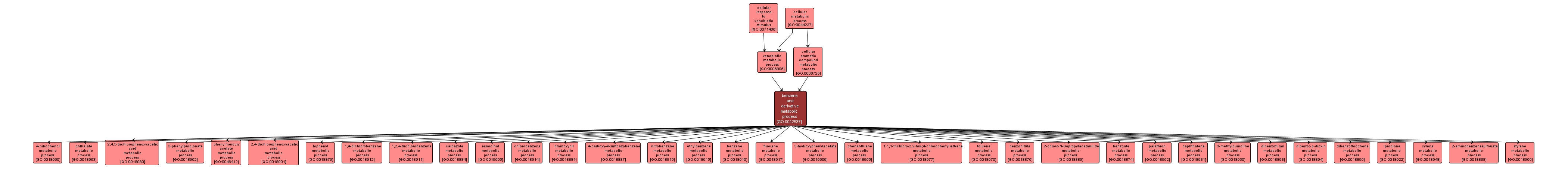
The chemical reactions and pathways involving benzene, C6H6, a volatile, very inflammable liquid, contained in the naphtha produced by the destructive distillation of coal, from which it is separated by fractional distillation, or any of its derivatives. |