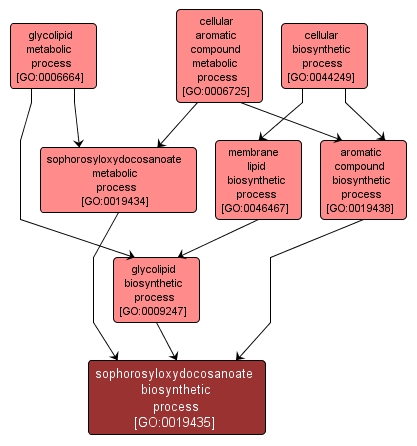
GO TERM SUMMARY
|
| Name: |
sophorosyloxydocosanoate biosynthetic process |
| Acc: |
GO:0019435 |
| Aspect: |
Biological Process |
| Desc: |
The chemical reactions and pathways resulting in the formation of sophorosyloxydocosanoate, 13-sophorosyloxydocosanoate 6',6''-diacetate. |
Synonyms:
- sophorosyloxydocosanoate synthesis
- sophorosyloxydocosanoate biosynthesis
- sophorosyloxydocosanoate anabolism
- sophorosyloxydocosanoate formation
|
|

|
INTERACTIVE GO GRAPH
|