GO TERM SUMMARY
|
| Name: |
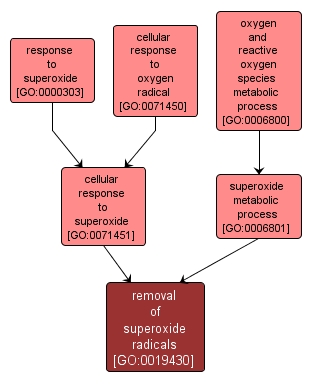
removal of superoxide radicals |
| Acc: |
GO:0019430 |
| Aspect: |
Biological Process |
| Desc: |
Any process involved in removing superoxide radicals (O2-) from a cell or organism, e.g. by conversion to dioxygen (O2) and hydrogen peroxide (H2O2). |
Synonyms:
- removal of oxygen free radicals
- removal of O2-
|
|

|
INTERACTIVE GO GRAPH
|