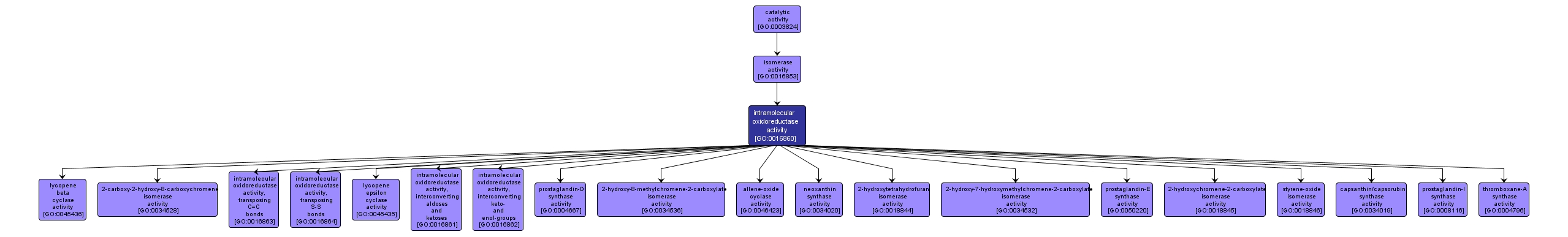
GO TERM SUMMARY
|
| Name: |
intramolecular oxidoreductase activity |
| Acc: |
GO:0016860 |
| Aspect: |
Molecular Function |
| Desc: |
Catalysis of an oxidation-reduction (redox) reaction in which the hydrogen donor and acceptor are the same molecule, and no oxidized product appears. |
Synonyms:
- intramolecular isomerase activity
- intramolecular oxidoreductase activity, other intramolecular oxidoreductases
|
|

|
INTERACTIVE GO GRAPH
|