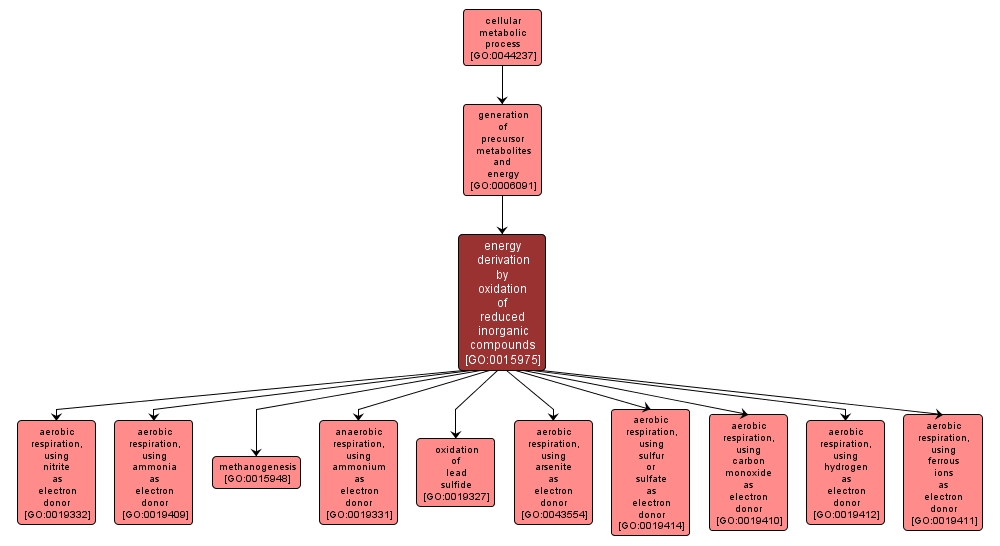
GO TERM SUMMARY
|
| Name: |
energy derivation by oxidation of reduced inorganic compounds |
| Acc: |
GO:0015975 |
| Aspect: |
Biological Process |
| Desc: |
The chemical reactions and pathways by which a cell derives energy from inorganic compounds; results in the oxidation of the compounds from which energy is released. |
Synonyms:
- chemolithotrophy
- lithotrophy
- chemolithotrophie
|
|

|
INTERACTIVE GO GRAPH
|