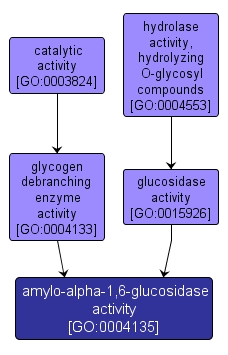
GO TERM SUMMARY
|
| Name: |
amylo-alpha-1,6-glucosidase activity |
| Acc: |
GO:0004135 |
| Aspect: |
Molecular Function |
| Desc: |
Catalysis of the hydrolysis of (1,6)-alpha-D-glucosidic branch linkages in glycogen phosphorylase limit dextrin. Limit dextrin is the highly branched core that remains after exhaustive treatment of glycogen with glycogen phosphorylase. It is formed because these enzymes cannot hydrolyze the 1,6 glycosidic linkages present. |
Synonyms:
- dextrin 6-alpha-D-glucosidase activity
- dextrin-1,6-glucosidase activity
- amylo-1,6-glucosidase activity
- amylopectin 1,6-glucosidase activity
- glycogen phosphorylase-limit dextrin alpha-1,6-glucohydrolase activity
|
|

|
INTERACTIVE GO GRAPH
|