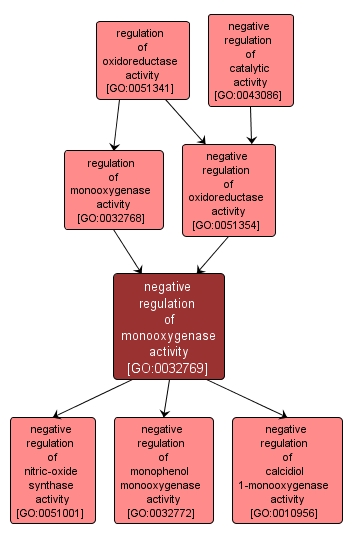
GO TERM SUMMARY
|
| Name: |
negative regulation of monooxygenase activity |
| Acc: |
GO:0032769 |
| Aspect: |
Biological Process |
| Desc: |
Any process that stops or reduces the activity of a monooxygenase. |
Synonyms:
- down-regulation of monooxygenase activity
- inhibition of monooxygenase activity
- downregulation of monooxygenase activity
- down regulation of monooxygenase activity
|
|

|
INTERACTIVE GO GRAPH
|