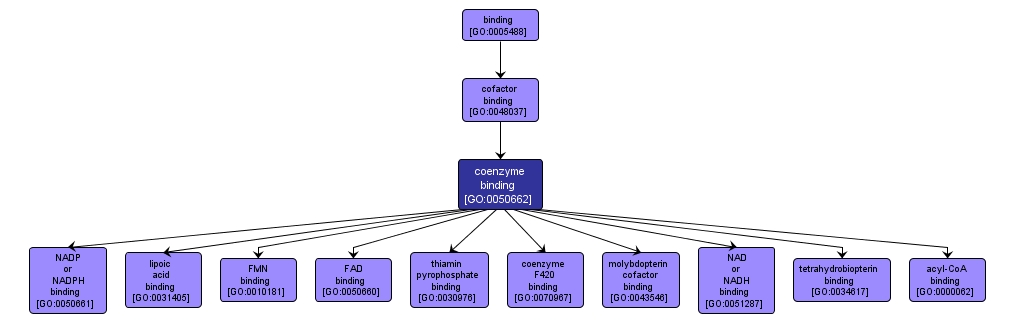
GO TERM SUMMARY
|
| Name: |
coenzyme binding |
| Acc: |
GO:0050662 |
| Aspect: |
Molecular Function |
| Desc: |
Interacting selectively and non-covalently with a coenzyme, any of various nonprotein organic cofactors that are required, in addition to an enzyme and a substrate, for an enzymatic reaction to proceed. |
|

|
INTERACTIVE GO GRAPH
|