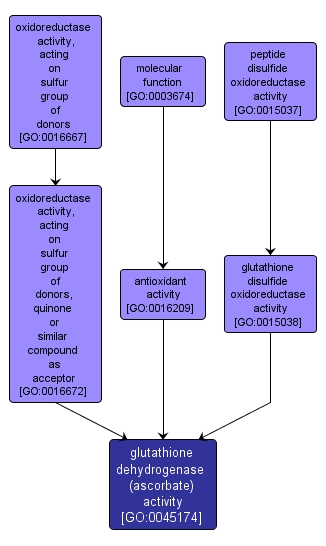
GO TERM SUMMARY
|
| Name: |
glutathione dehydrogenase (ascorbate) activity |
| Acc: |
GO:0045174 |
| Aspect: |
Molecular Function |
| Desc: |
Catalysis of the reaction: 2 glutathione + dehydroascorbate = glutathione disulfide + ascorbate. |
Synonyms:
- dehydroascorbic acid reductase activity
- GDOR
- dehydroascorbic reductase activity
- glutathione dehydroascorbate reductase activity
- glutathione:dehydroascorbate oxidoreductase activity
- dehydroascorbate reductase activity
- DHA reductase activity
- glutathione:dehydroascorbic acid oxidoreductase activity
|