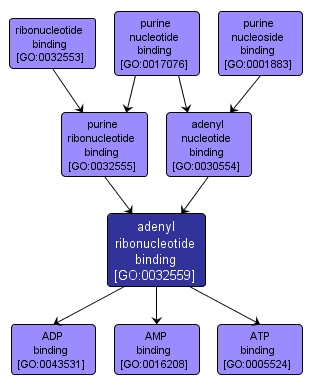
GO TERM SUMMARY
|
| Name: |
adenyl ribonucleotide binding |
| Acc: |
GO:0032559 |
| Aspect: |
Molecular Function |
| Desc: |
Interacting selectively and non-covalently with an adenyl ribonucleotide, any compound consisting of adenosine esterified with (ortho)phosphate or an oligophosphate at any hydroxyl group on the ribose moiety. |
|

|
INTERACTIVE GO GRAPH
|